The Obama administration, in an effort to forge ahead with its sometimes-contentious effort to compare various medical treatments, is proposing a big boost in funding for the agency that oversees the research.
Proponents say the research can provide patients and their doctors with crucial information to help them decide among various drugs or treatments. Critics, on the other hand, say the research could be used to limit or ration care if the federal government or insurers used the information to deny coverage for a particular test or procedure because it was found to be less effective.
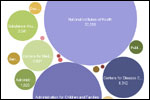
The administration, releasing its 2011 budget request to Congress on Monday, proposed spending $286 million on comparative effectiveness research overseen by the Agency for Healthcare Research and Quality. The agency got $21 million for such research in its current fiscal-year budget, and an additional $300 million for such research in the economic stimulus bill.
Efforts to compare drugs or treatments are not new; private insurers and Medicare consider medical evidence at times in a bid to determine whether a new treatment works well for particular types of patients. But the research is often controversial.
In December, for example, a recommendation from a government task force dealing with annual mammograms for women under age 50 raised concerns among advocacy groups and lawmakers that it might lead to restrictions on mammograms for some women.
In the fiscal 2011 budget document for the Department of Health and Human Services, officials used the term “patient-centered health research” rather than “comparative effectiveness,” a shift some analysts said was done to distance the issue from its somewhat painful past on Capitol Hill.
Both the House and Senate health care overhaul bills now stalled in Congress would also boost funding for comparative effectiveness research.
The House legislation would provide $300 million over three years for the research, according to an analysis from Avalere Health, a Washington-based consulting firm. The Senate proposed creating a nonprofit, nongovernmental agency to oversee the research effort, spending $165 million over three years, according to the analysis.
In an interview Monday, AHRQ Director Carolyn Clancy said if Congress approves the president’s request, the funding would be used to continue the agency’s research in 14 different areas, including cancer, obesity and substance abuse.
“We’re going to continue the work we’re doing now to frankly fill the huge gaps in information that clinicians and patients face every day,” Clancy said. “It’s almost a by-product of how well we’ve done in biomedical science that for many, many decisions, diagnosis and treatment, patients and clinicians have two or more options. What they don’t have is good, comparative information that helps them figure out that for this individual what’s the right choice to make.”
Tony Coelho, chairman of the Partnership to Improve Patient Care, a group that opposes using comparative effectiveness research for insurance-coverage decisions, said patients and physicians must be involved in any federal efforts in comparative effectiveness research and that Congress should closely track how the money is spent.
“As this provision goes forward I would think a lot of members in the House and the Senate would want to know a little bit more about what the administration has in mind and how they want to spend this additional money as opposed to what they’re doing with the money they already have,” Coelho said.
Former Centers for Medicare and Medicaid Services Administrator Mark McClellan said while many health care groups support the intent of comparative effectiveness research, “the next round of the debate is whether there is a way for Congress to comfortably support developing better evidence to guide individuals’ decisions without raising this fear of controlling those decisions.Whichever side of the debate you’re on, we need better evidence.” McClellan now directs the Engelberg Center for Health Care Reform at the Brookings Institution.